Wednesday, December 9, 2009
Electrochemistry Review Solutions
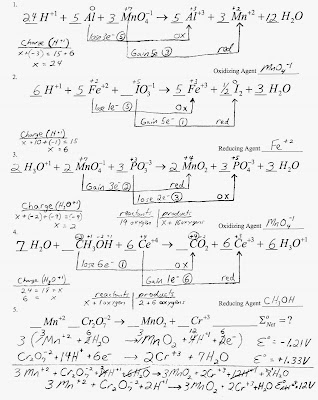
Below are the solutions for the test on Electrochemistry. Make sure to also look at the balancing redox reactions problems on the previous post. You will be given a half cell reduction table and all of the rules for assigning oxidation numbers and balancing complex redox reactions.


Friday, December 4, 2009
Below are practice problems for balancing complex oxidation-reduction reactions. Problems 1-4 are only balancing problems, thus use the method that was introduced on part (c) of Balancing Redox Reactions worksheet. Problem #5 asks for a net voltage. You need to find half reactions that fit the products and reactants given in the equation. You will notice that no water or hydrogen ions are given. The correct half reactions will supply the waters and hydrogens needed for the entire redox reaction. One word of advice, like "species" on each side of the yield sign will factor each other out. The solutions to each problem are given at the end of the blog entry. Try the problems before you look at the solutions.


Below is the key for the redox problems.
Subscribe to:
Comments (Atom)